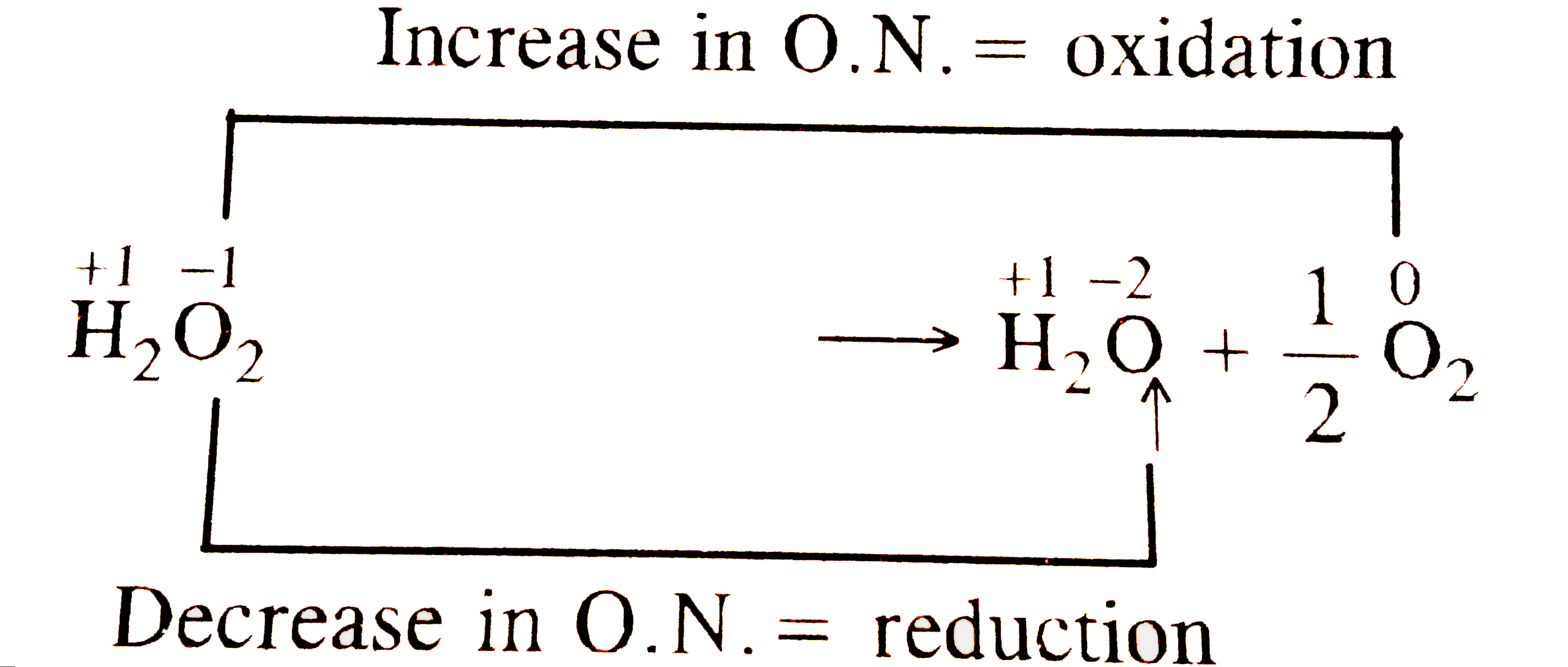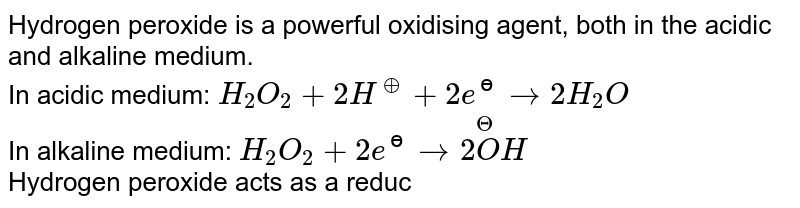Oxidation and reduction – oxygen transer A substance has been oxidised if it gains oxygen. Oxidation is gain of oxygen. A substance has been reduced if. - ppt download
Show how H2O2 functions both as a reducing agent and as a oxidizing agent? - Sarthaks eConnect | Largest Online Education Community

An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. - YouTube

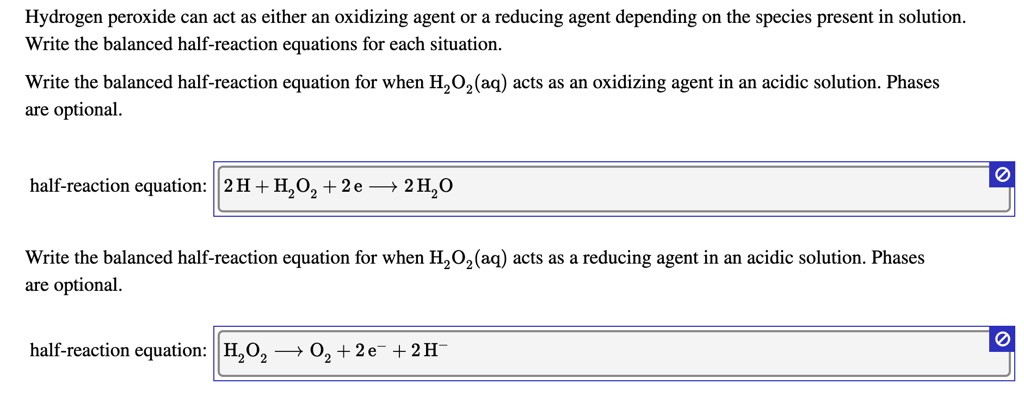
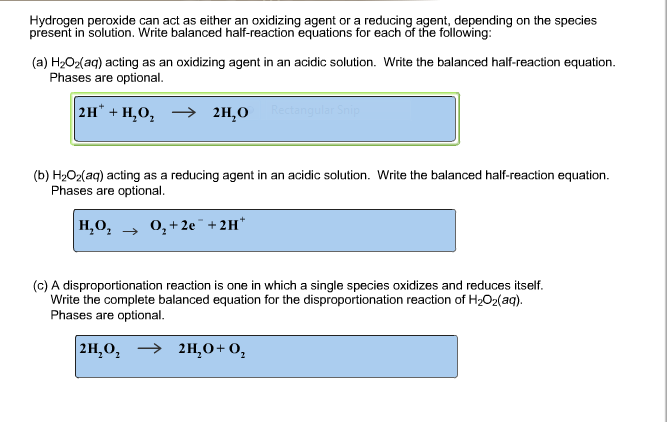
Hydrogen peroxide can act as either an oxidizing agent or a reducing agent, depending on the species present in solution. Write balanced half-reaction equations for each of the following: (a) H2O2(aq)
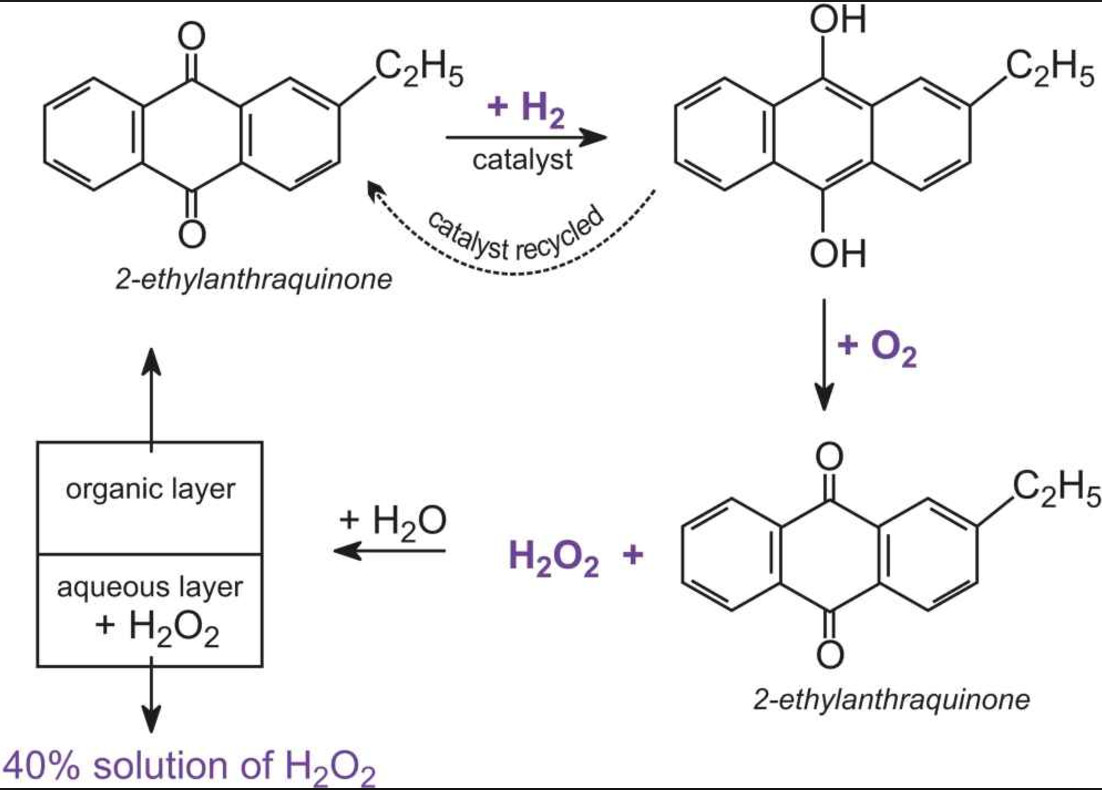
How will you show that H2O2 acts as both oxidising and reducing agent? What is meant by '30 volume' of H2O2? - Quora

While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their - Brainly.in

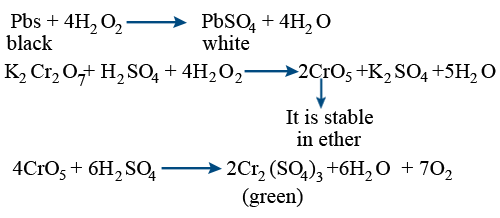
Write the chemical reaction to justify that hydrogen peroxide can function as an oxidising as well as - Brainly.in
An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. Illustrate it with the help of a chemical equation. - Sarthaks eConnect | Largest Online Education Community